You have no doubt seen gal-vanised steel articles such as buckets and wheelbarrows, and will have seen how long they last before rust sets in, even when dumped outside in the garden.
The great point is that the protection continues even when the surface is scratched or otherwise damaged, unlike a paint layer. Car manufacturers have made the same observation, and are now making use of more and more galvanised steel sheet.
Galvanising involves dip-ping steel into molten zinc. Unfortunately, this is not a practical option for the home workshop as the zinc fumes are poisonous. There are other processes such as Sherardizing, but these are equally unsuitable for us. So we need to use elec-troplating.
Briefly, the principle behind electroplating is the deposition of a metal layer on a base metal, using direct current through a solution called an electrolyte. Zinc is one of the easier metals to plate. It gives good corrosion protection, as we have seen, and can be pol-ished to give a finish almost as shiny as chromium. No exotic chemicals are needed (unlike other industrial plating processes which use cyanide salts).
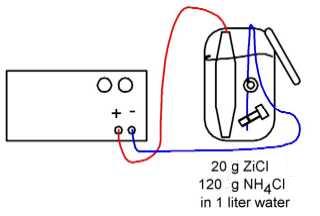
The first requirement for plating is that your compo-nents are truly clean. The best way to ensure this is by some sort of degreasing, using solvent or even better, scrubbing with caustic soda. Follow this with what is known as a 'pickle'. You will need something stronger than vinegar, though - use hydrochloric acid. It is available as Spirits of Salts from an ironmongers. It needs to be diluted to a 20% solution. Hydrochloric acid is very corrosive, so be careful. Use gloves and goggles, and follow the normal rule when diluting acids: add acid to water, not water to acid. Used at room temperature, the pickle takes about four minutes to clean steel parts effectively. Wash the parts well under a tap, but don't touch them afterwards, otherwise you will have to start again. For the plating set-up, this is what you will need: # A suitable sized plastic or stainless steel container. # A dc electrical source, e.g. a car battery or charger, with an ammeter to check current levels. # A small quantity of pure zinc; You might be able to buy something called 'perforated zinc' (i.e. zinc sheet with lots of tiny holes) at an old-fashioned ironmongers. Alternatively, look up non-ferrous scrapyards in Yellow Pages. 1 bought about 5kg (much more than 1 could ever use) for £1.70 at my local dealers. # For the electrolyte - zinc chloride and ammonium chloride. You can get this from chemistry set suppliers, or good pharmacists; or lookup 'Chemical Manufacturers and Suppliers' in Yellow Pages. To make up one litre of the electrolyte, dissolve 120 grams of ammonium chloride in 750 ml of tap water. Then dissolve 20 grams of zinc chloride in 250 ml of warm tap water. Then carefully mix the two together. You will, of course, once again take the usual precautions with these chemicals, wearing gloves and goggles. Zinc chloride solution is corrosive (it can be used as a flux for soldering, when it is known as Baker's Fluid, by the way). You will need to have some way of controlling the current to your plating bath. If you have a model railway controller or similar that would be perfect. Otherwise if you are using a car battery as a power source, and can get hold of a rheostat, that would be OK. The current needs to be kept to around 100 milliamps per square-inch of surface to be plated. The zinc will be the anode.' so this is connected to the positive terminal. The workpiece is the cathode, connected to the negative terminal. Adjust the current to the correct level, and see what happens. Before long, you will see your component turning grey: this is the zinc layer forming. The longer you leave it, the thicker the layer. You might like to experiment with the current levels, as the greater the current, the faster the zinc is deposited. However, if the current is too high, the deposit is rough and hence no good. You will need to experiment to find out what parameters are best for your set-up. When the plating is complete, scrub the component under the tap, and let it dry. Then you can polish it up. With any luck, you should have a finish almost as shiny as chrome plate, but much more durable. Here's the diagram:
